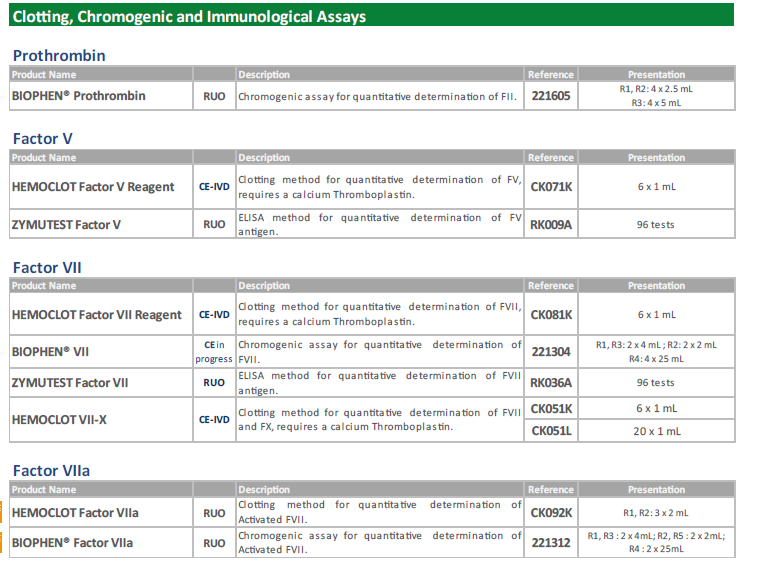
BioMed 常用的發(fā)色底物
法國(guó)HYPHEN BioMed公司(簡稱HBM)--歐洲知名血液産品生産商,通過(guò)國(guó)際ISO9001和ISO13485雙項認證,生産的大部分産品均通過(guò)歐洲CE認證,爲臨床診斷提供了可靠的保證.
法國(guó)HYPHEN BioMed公司是一家生産血栓止血,凝血纖溶和自身免疫的診斷試劑廠家。其産品包括:血凝試劑,發(fā)色底物,ELISA試劑盒,人和動物的純化酶及純化蛋白,單克隆和多克隆抗體.應用手工或者自動化的方法,爲您的實驗室診斷、免疫學(xué)研究,提供一系列完整的成(chéng)套試劑,并廣泛應用于生物技術和醫産業。
BIOPHENTM Factor VIIa
REF 221312
R1 R3 2 x 4 mL, R2 2 x 2 mL, R4 2 x 25 mL
Quantitative determination of Factor VIIa activity, in purified medium, using chromogenic assay.
FOR RESEARCH USE ONLY.
DO NOT USE IN DIAGNOSTIC PROCEDURES.
INTENDED USE:
The BIOPHEN™ Factor VIIa kit is a chromogenic method intended for in vitro quantitative determination of activated Factor VII (FVIIa) activity, in purified milieu, using manual or automated method.
This kit is for research use only and must not be used for patient diagnosis or treatment.
PRINCIPLE:
Factor VII is the serine esterase of the extrinsic coagulation pathway. When complexed to Tissue Factor (TF), in presence of phospholipids and calcium, it activates Factor X to Factor Xa.
The BIOPHEN™ Factor VIIa kit is insensitive to Factor VII.
Factor VIIa forms an enzymatic complex with recombinant truncated human Tissue Factor (rTTF) and synthetic phospholipids. It then activates Factor X, present in the assay at a constant concentration and in excess, to Factor Xa. The FXa activity is measured using a specific chromogenic substrate (SXa-11). Factor Xa cleaves the substrate and generates pNA. The amount of pNA generated is directly proportional to the Factor Xa activity. Finally, there is a direct relationship between the amount of Factor VIIa in the assayed sample and the Factor Xa activity generated, measured by the amount of pNA released, determined by colour development at 405 nm.

 010-64814275
010-64814275
 當前位置:
當前位置:




 010-64814275
010-64814275
 010-64814275
010-64814275
 2850669802
2850669802